Basics
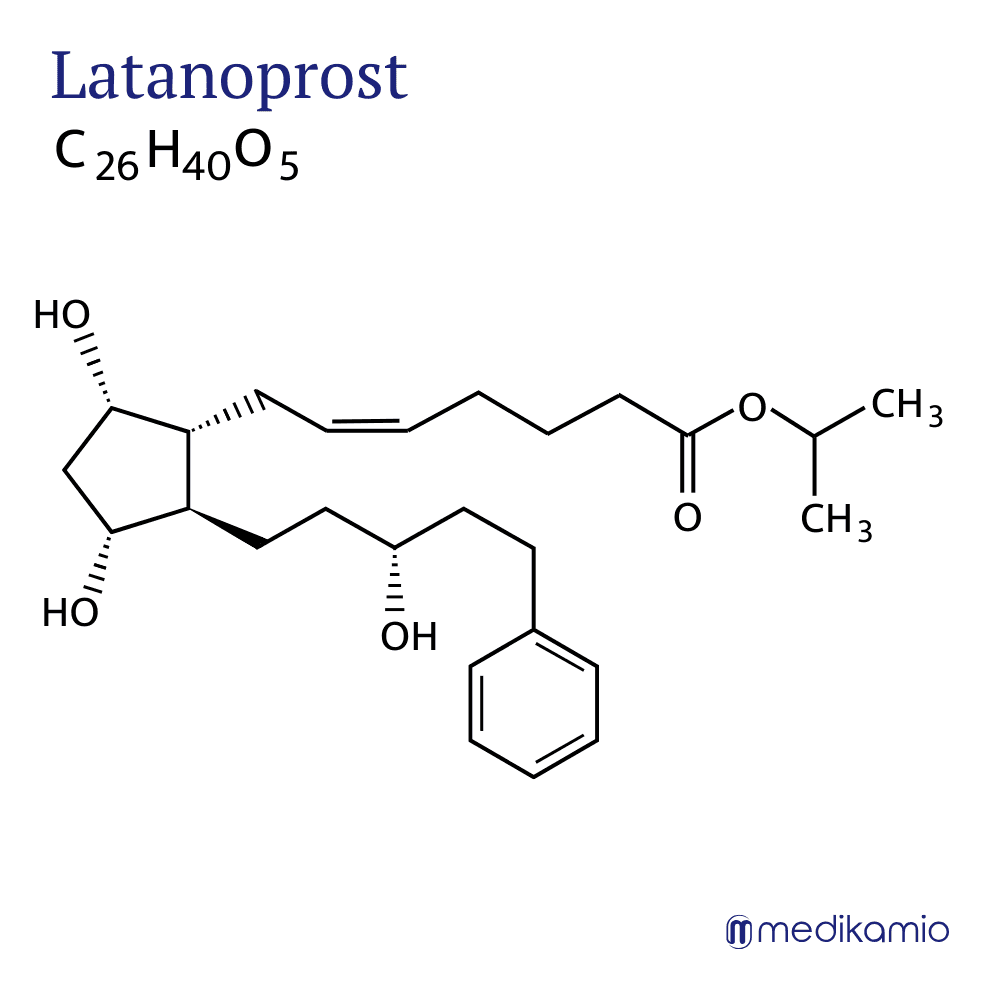
Latanoprost is an active ingredient for lowering intraocular pressure in glaucoma (open-angle glaucoma) and in cases of increased intraocular pressure. It belongs to the group of prostaglandin analogs. Latanoprost is a prostaglandin F2-alpha derivative and is present as a slightly yellowish oil. It is insoluble in water and a prodrug that is converted to active latanoprost acid and isopropanol in the cornea of the eye. Latanoprost is available in the form of eye drops. Depending on the preparation, the active ingredient must be stored in a cool place or at room temperature.