Basics
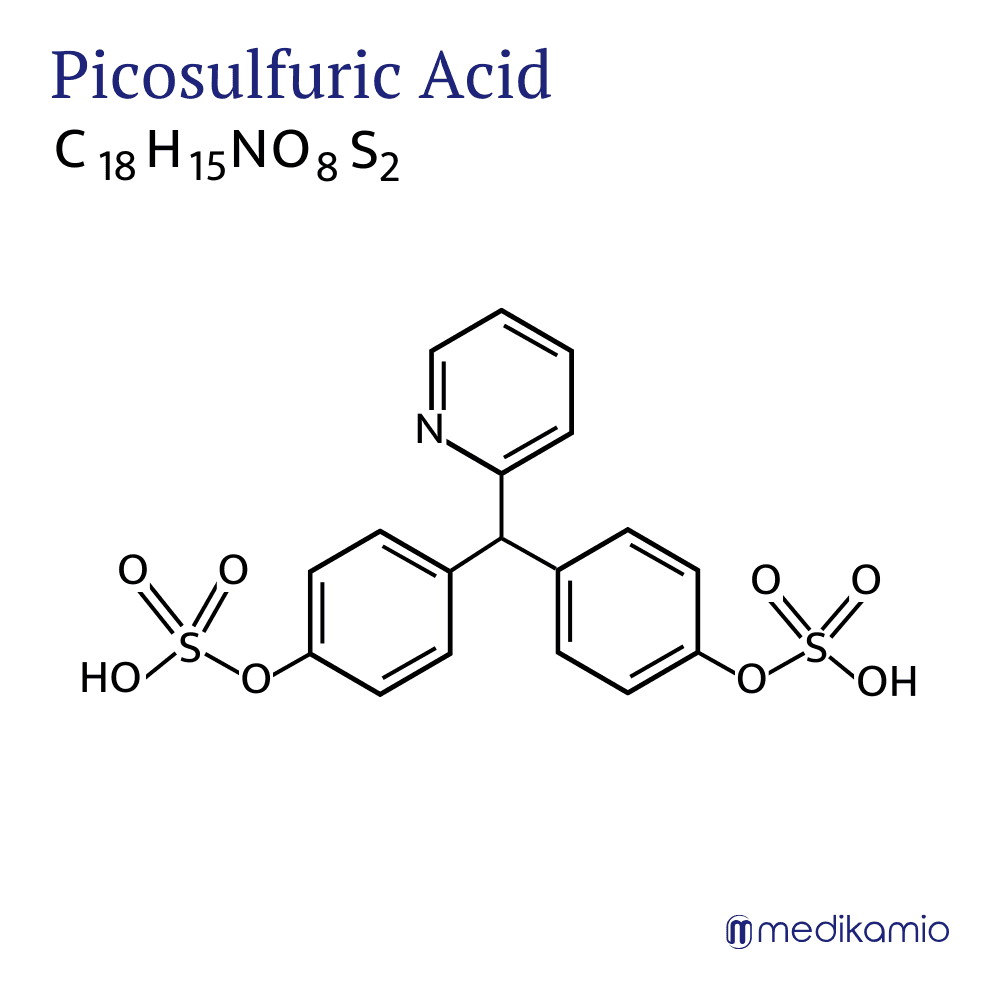
Sodium picosulphate is an active ingredient that is used to treat constipation and to empty the bowels. It belongs to the group of laxatives. It is very closely related to bisacodyl and is usually available as a white crystalline powder. Sodium picosulphate is a prodrug, which means that the active ingredient is present as an inactive form and is only converted into the active form, the diphenol bis-(p-hydroxyphenyl)-pyridyl-2-methane (BHPM), in the body through the conversion processes. The conversion is carried out by certain intestinal bacteria. The advantage of sodium picosulphate is that it does not need to be present in enteric-coated form because it is not absorbed.