Basics
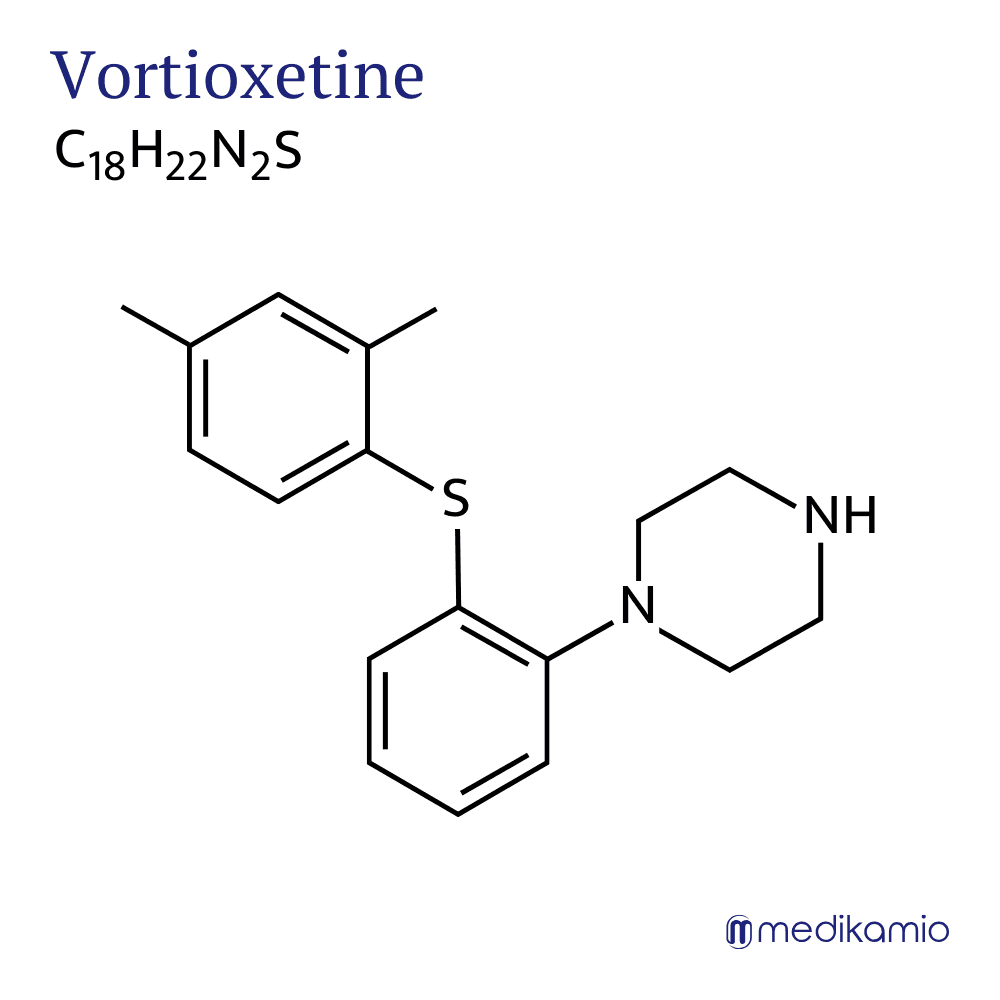
Vortioxetine is an active ingredient for the treatment of depression and anxiety disorders. It belongs to the group of serotonin antagonist and reuptake inhibitors (SARI). Vortioxetine is available as a tablet or solution. It is a piperazine derivative and is usually available as vortioxetine hydrobromide. It is also a white to beige powder that is only slightly soluble in water. As a solution, the active ingredient is usually available as vortioxetine DL-lactate.