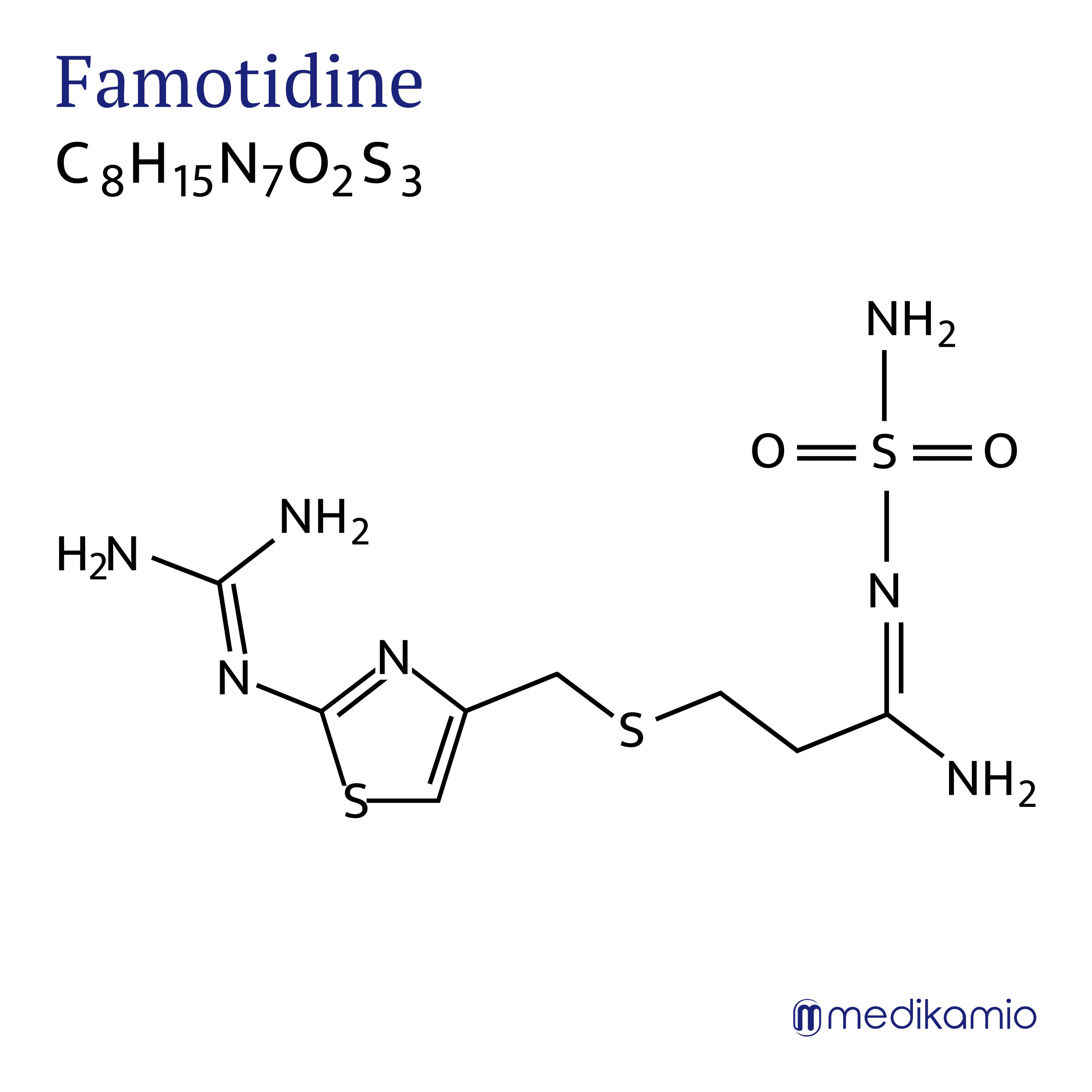
Famotidine is a competitive histamine-2 (H2) receptor antagonist. This means that it blocks the H2 receptor (antagonist) as long as the concentration of famotidine is higher than that of the substances that normally bind to this receptor (competitive). It is used to inhibit the secretion of gastric acid and the enzyme pepsin. Famotidine has the advantage that it binds very specifically to the H2 receptor (selective) and therefore causes relatively few side effects. It is also stronger (more potent) than other drugs used for the same therapy.
Famotidine is mainly broken down via the liver, but is also excreted unchanged via the kidneys to a large extent. The bioavailability of famotidine - i.e. the percentage of the active ingredient available in the blood - is 40%. The half-life, i.e. the time the body needs to excrete half of the active substance, is approx. 3 hours. The maximum plasma concentration (Cmax), i.e. the maximum concentration of the active substance in the blood plasma (liquid cell-free part of the blood) is reached approx. 1-3.5 hours after ingestion.