Basics
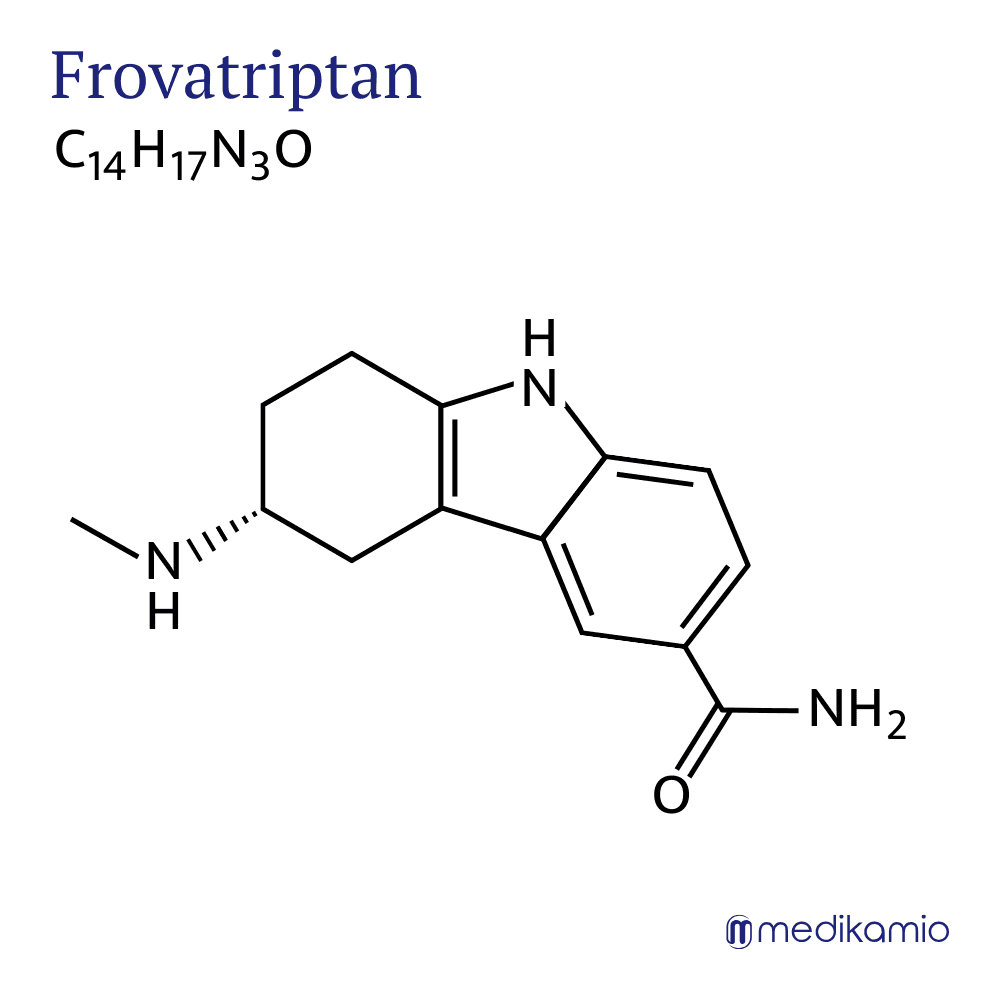
Frovatriptan is an active ingredient for the acute treatment of migraine attacks. It is a vasoconstrictive agent and belongs to the group of triptans. It is available in tablets and is usually in the form of frovatriptan succinate monohydrate. It is also a white powder that is soluble in water. Frovatriptan is an indole derivative that is structurally related to serotonin.