Basics
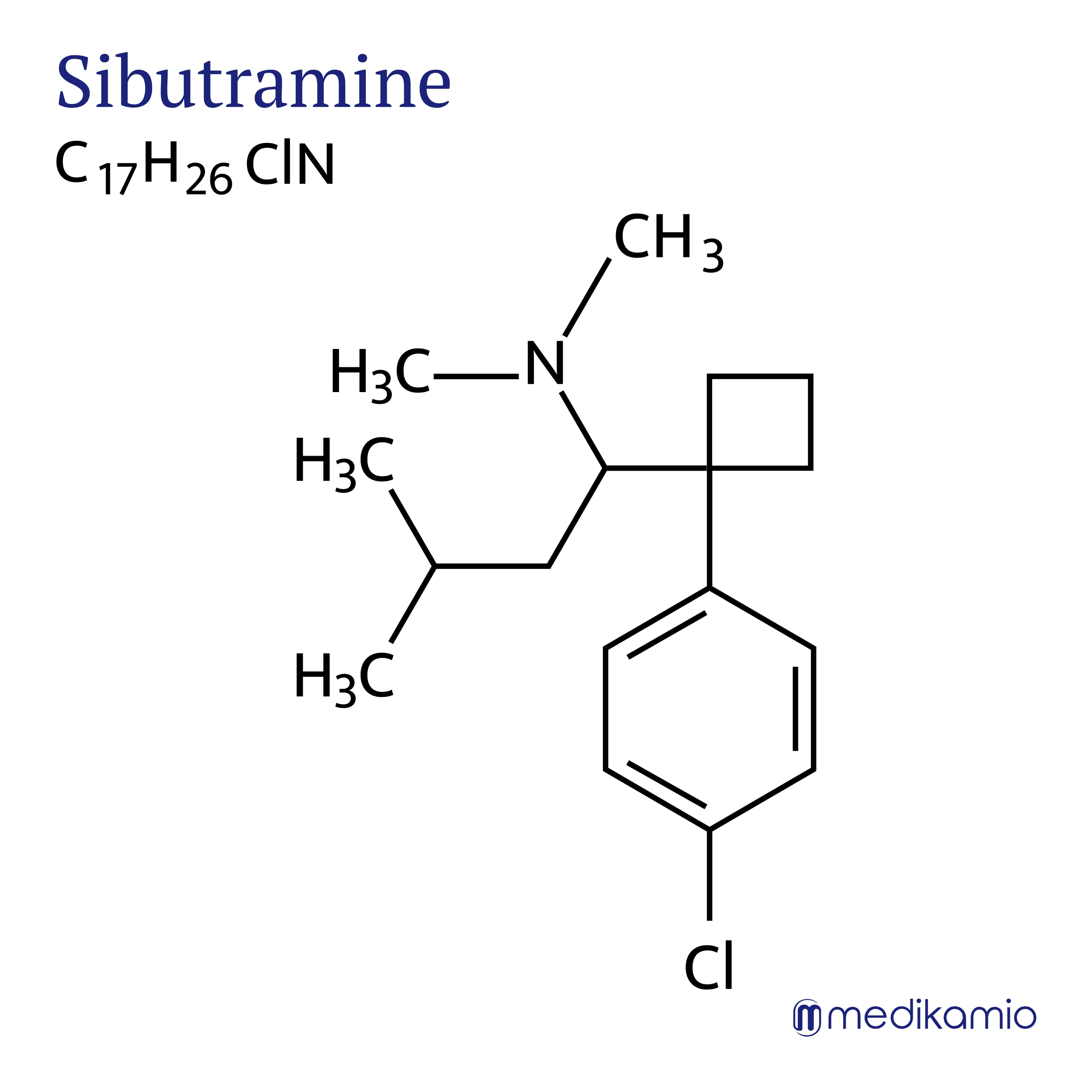
Sibutramine is an active ingredient that is used to treat overweight (obesity). The active ingredient is used in combination with diet, behavioral changes and exercise. It belongs to the anorectic group and is a serotonin-norepinephrine reuptake inhibitor and is therefore closely related to antidepressants. In 2010, the European Medicines Agency (EMA) decided to withdraw the active ingredient from the market due to severe side effects affecting the cardiovascular system. Sibutramine causes an increased risk of suffering a heart attack or stroke and can therefore have fatal side effects. In addition, the EMA reassessed the benefit-risk profile and came to the conclusion that patients who took sibutramine only lost 2-4 kg more weight. This effect was too small for the EMA to keep the drug on the market. In addition, the effect stops when you stop taking sibutramine.