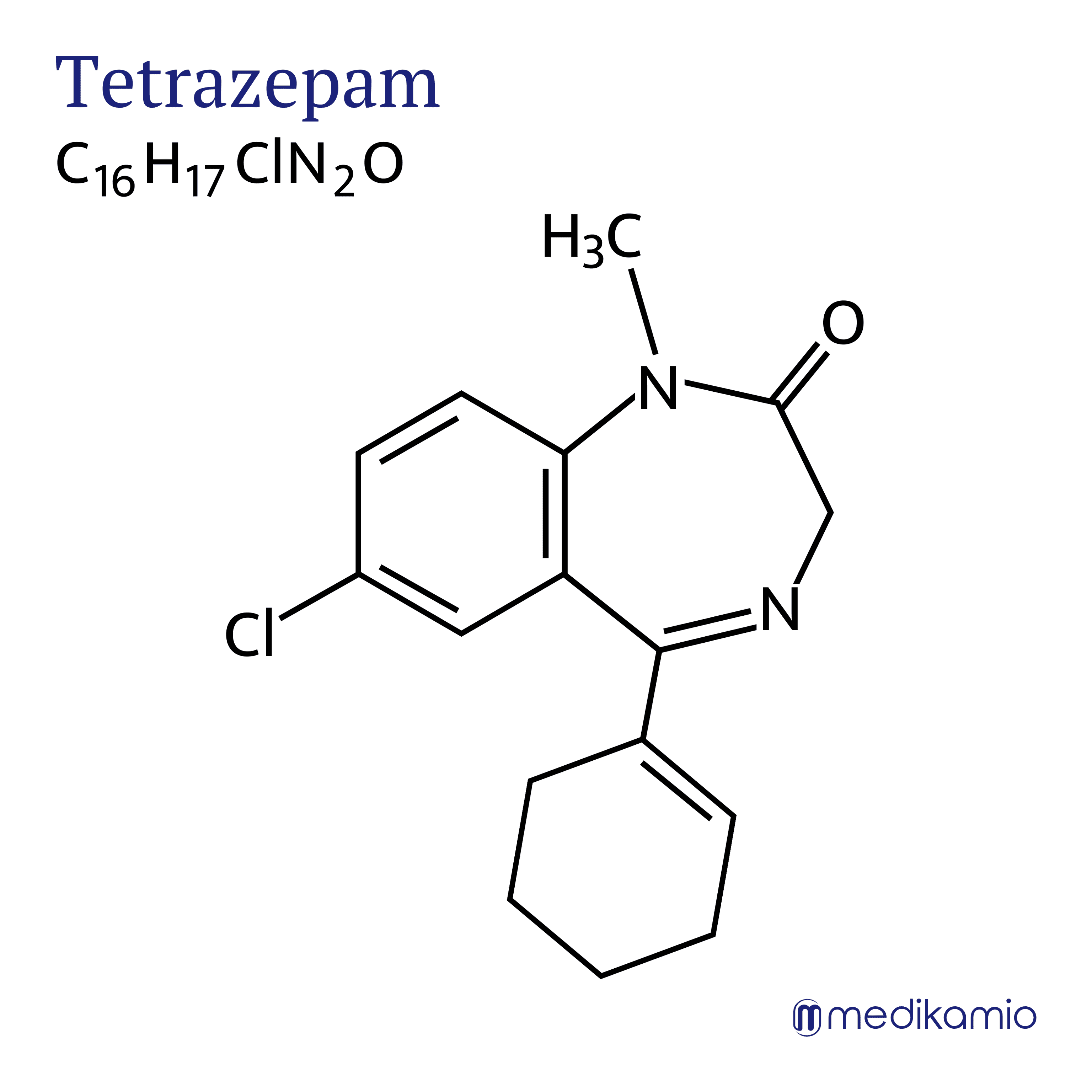
Tetrazepam is used as a muscle relaxant, especially for seizures or chronic pain caused by muscle tension. There are more suitable drugs from the benzodiazepine group for use as a sedative, anxiolytic or sleep-inducing medication.
Tetrazepam acts on the GABA-A receptors and increases the effect of the neurotransmitter GABA when it is present. This receptor binding results in an increased influx of chloride ions, which in turn leads to an increased electrical voltage in the cell (hyperpolarization). This effect protects the cell from overexcitation. Tetrazepam is GABA-dependent, which means that tetrazepam can only work if GABA binds to the same receptor, i.e. they must bind to the receptor at the same time. The advantage of this is that the risk of respiratory depression, i.e. respiratory arrest due to muscle paralysis, is reduced compared to drugs used in the past. Tetrazepam leads relatively quickly to habituation, which can lead to dependence.
Tetrazepam is absorbed via the gastrointestinal tract and is 83-87% bound to plasma proteins after ingestion by tablet. The maximum plasma concentration is reached after 1.5-2 hours. Tetrazepam is metabolized in the liver and excreted almost exclusively via the kidneys. A steady-state condition is reached after approx. 3 days, which means that the full effect is only achieved after a constant intake of the drug for 3 days.