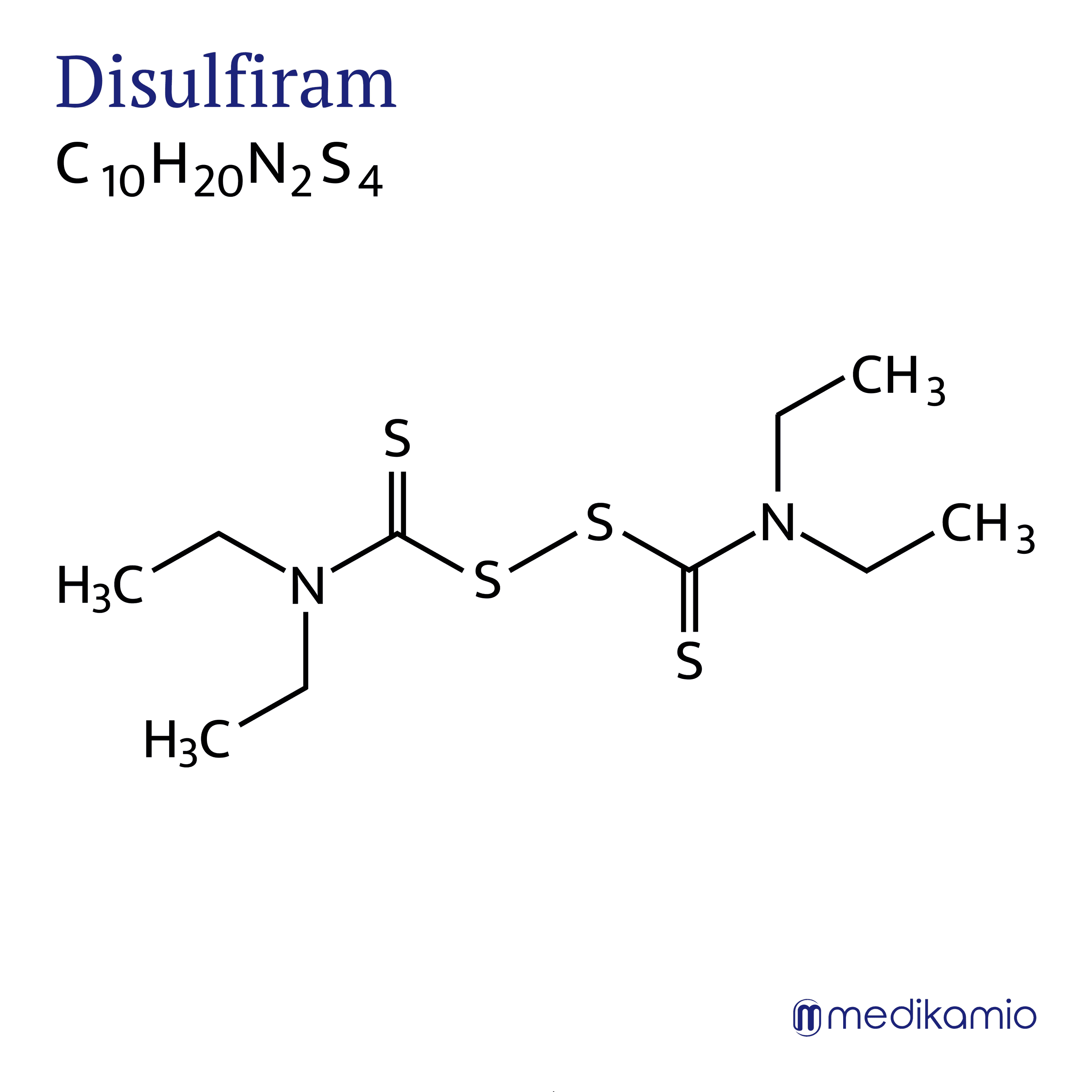
During disulfiram therapy, the intake of alcohol should be avoided at all costs, as otherwise fatal (lethal) complications such as a heart attack (myocardial infarction) or angina pectoris may occur. Intolerance reactions occur from a quantity of 3 g of pure alcohol (ethanol), which corresponds to approx. 75 ml of beer or 32 ml of wine. It should be noted that alcohol is also contained in some medicines and foods (cough syrups, tinctures, chocolates with alcohol content, etc.). In any case, those affected should be prepared to abstain from alcohol.
Interactions may occur if the following medicines are taken at the same time:
Disulfiram can increase the effect of the following medicines:
- Benzodiazepines (sleeping pills and tranquillizers, anti-anxiety medication)
- Phenytoin (medication for epilepsy)
- Clomipramine (mood-enhancing medication)
- Pimozide (medication for mental illnesses)
- Rifampicin and isoniazid (tuberculosis medication)
- Medication to inhibit blood clotting ("blood thinners")
- Metronidazole (medication for infections)
- Theophylline (medication for respiratory diseases)
The following medications increase intolerance reactions:
- Cyanamide
- metronidazole
- tricyclic antidepressants
- MAO inhibitors
- chlorpromazine
- phenothiazine compounds
- antihypertensive drugs (vasodilators, alpha and beta receptor blockers)
Drugs for the treatment of allergies (antihistamines) and diazepam can weaken the intolerance reactions.
Disulfiram should not be taken with aldehyde-containing medications.
Drugs to neutralize stomach acid (antacids) can reduce the absorption of disulfiram.